Register here to download the data of the challenge!
Data
The geometrical design of the simulation phantoms was done through an iterative optimization process starting from an overlapping set of large tubular fibres of 15 μm of diameter (or strands), with both endpoints located in regions of interest (ROIs) on the surface of a sphere of 1 mm in diameter. Each strand was set to follow a trajectory of an arc of a circle going through the centre of the sphere. Those strands were slowly separated using energy terms controlling for length, curvature, and overlap with other strands. This optimization was done using the Numerical fibre Generator (Close et al., 2009). Then, the tubular fibres were resampled with a smaller diameter (7.5 μm), and their trajectories re-optimized. Finally, each strand was further subdivided into strands which diameters following a gamma distribution Γ(κ, θ), with shape, κ=0.5, and scale θ=0.007 (minimum diameter of 2 μm and maximum diameter of 6 μ). Those strands were optimized for an additional iteration to further remove overlapping strands.
The final set of strands (trajectories and diameters) were used to generate a mesh of the substrate. For each strand, a tubular mesh was generated to represent the outer surface of the axon-like structure. An additional inner tubular mesh following the same trajectory but with a diameter of 0.7 times the outer diameter, representing the inner surface of the fibre. The meshes were used as input to the open-source MC/DC Monte Carlo Diffusion and Collision Simulator (Rafael-Patino et al., 2020). All particle initiated inside the inner-mesh were considered as “intra-axonal” water, those within the outer-mesh but outside the inner mesh were considered “myelin” water, and those outside the outer-mesh were considered “extra-axonal”. Particles initiated in the "extra-axonal" and "intra-axonal" compartments were used for the DW-MRI signal generation. The unrestricted diffusion coefficient of the Monte Carlo particles was set to 0.6 × 103 mm2/s. To ensure a high and uniform density in all the 64,000 computed voxels (image 40x40x40 voxels), we used a highly dense regular particle placement with a total of 109 particles to achieve a density of one particle per cubic micrometre and reduce simulation noise.
All datasets were generated using the same substrate optimization procedure and simulated using the same microstructure parameters and properties, but simply using a different seed for the random generation of connectivity matrices. Although the connectivity matrices have similar controlled sparsity levels, the distribution of the connectivity weights between pairs of ROIs in the matrices are different. This inevitably affected the strand trajectory optimization process done using the Numerical fibre Generator. Synthetic fibre diameters are also drawn from the same distribution for all datasets. The simulated images capture the microscopic properties of the tissue (e.g. fibre diameter, water diffusing within and around fibres, free water compartment), while also having desirable macroscopic properties resembling the anatomy, such as the smoothness of the fibre trajectories.
For all three phantoms, participants will have access to the noisy diffusion signal for various diffusion acquisition parameters, a mask of the white matter volume, and a label map of the endpoint connectivity. The diffusion protocol includes 360 diffusion-weighted images and 4 non-diffusion-weighted images (b=0 s/mm2). The diffusion-weighted measurements are distributed over 4 b-shells (b=1000, 1925, 3094, 13191 s/mm2). Those correspond to the 3 b-shells of the ActiveAx protocol (Alexander et al., 2010) with an additional shell at b=1000 s/mm2 (echo time of 0.0535s). Each shell was sampled using 90 uniformly distributed gradient directions on the sphere. For the training phantom, participants will have access to the map of intra-/extra-fibre signal fractions, the noise-less diffusion signal, the ground truth connectivity matrix, the distribution of simulated fibre diameters and trajectories, and the evaluation script to train their connectivity estimation method. Participants will also have access to a validation dataset with its corresponding connectivity matrix to evaluate the performance of their method.
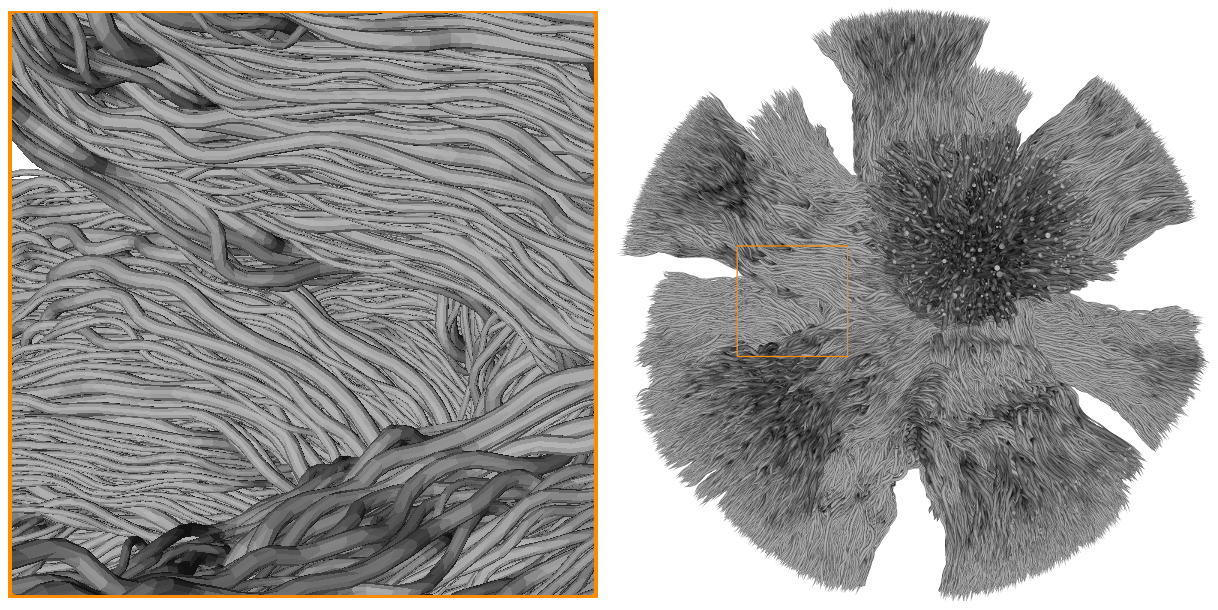
Mesh of the testing dataset used for the Monte-Carlo simulations of spins dynamics to generate the DiSCo DW-MRI dataset. The mesh is composed of 12,196 tubular fibres, with gamma-distributed outer diameters ranging from 2μm to 6 μm, connecting 16 ROIs located on the surface of a sphere of 1 mm in diameter.
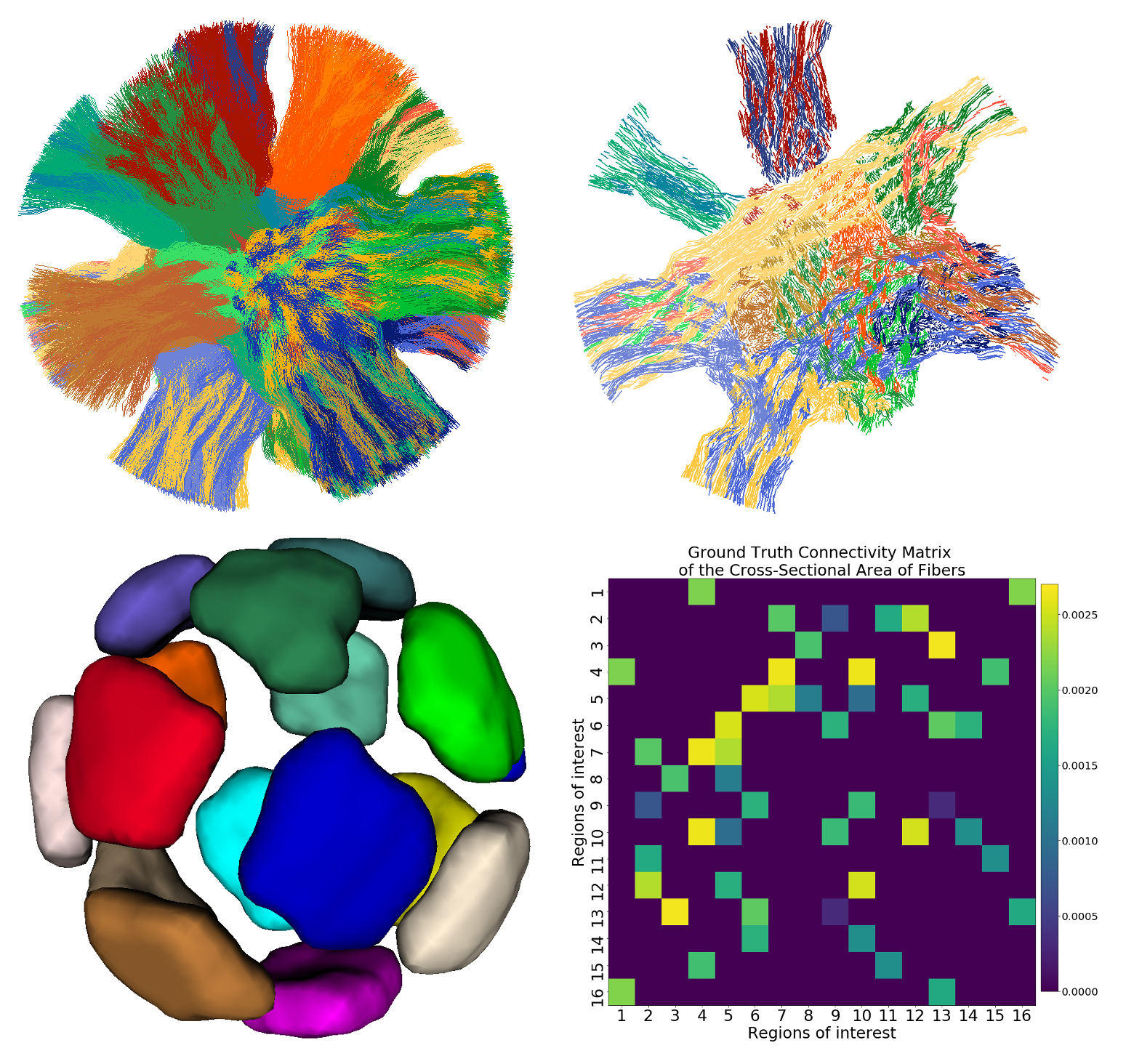
Three dimensional (top left) and cross-sectional (top right) views of the trajectory of the 12,196 tubular fibres. Trajectories sharing both endpoints are shown with the same color. The 16 ROIs and the ground truth connectivity matrix are shown on the left and right of the bottom row, respectively. The weights of the matrix corresponds to the sum of the cross-sectional area of the tubular fibres connecting each pair of ROIs.
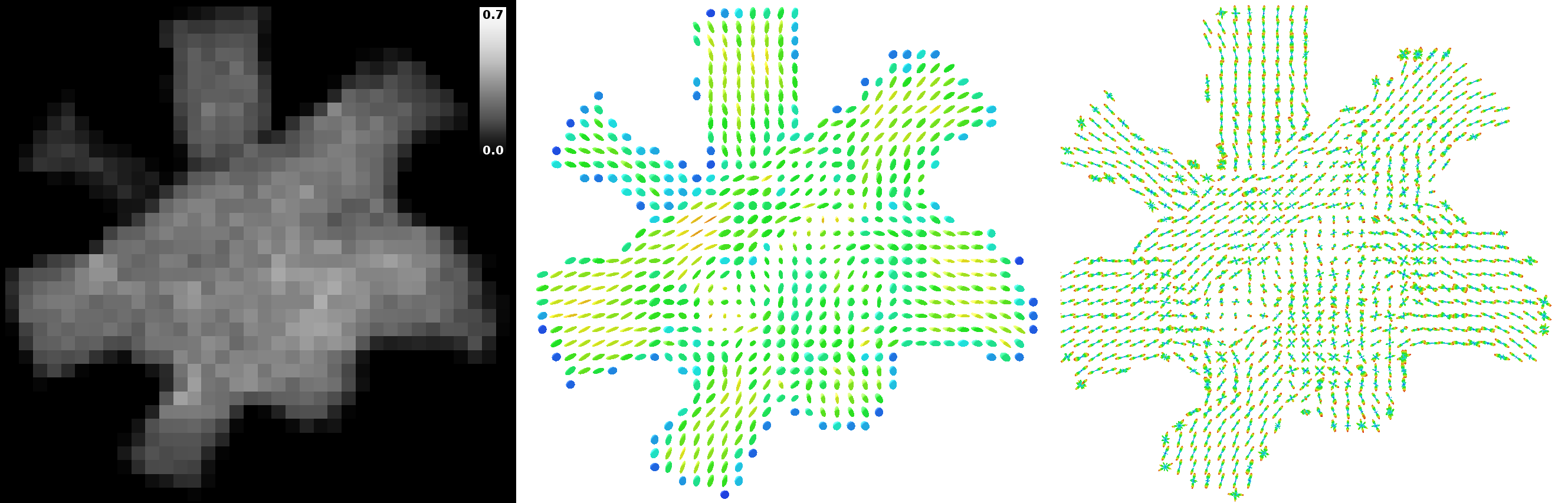
“Intra-axonal” volume fraction map of the center of the phantom (left), and corresponding diffusion tensors (center) and fibre orientation distributions (right). The “intra-axonal” volume fraction corresponds to the fraction of samples initiated inside the inner diameter of the tubular fibres, over all samples used to generate the DW-MRI synthetic signal. Diffusion tensors and fibre orientation distributions were estimated using the noiseless DW-MRI signal.
Ground truth trajectories (left) and example of estimated trajectory using probabilistic streamline tractography (right). The first row shows the five existing connections of ROI 10, the bottom row shows the four existing connections of ROI 4. ROIs 4 and 10 are connected by the light green trajectories.
References
Rafael-Patino, J., Romascano, D., Ramirez-Manzanares, A., Canales-Rodríguez, E. J., Girard, G., & Thiran, J. P. (2020). Robust Monte-Carlo Simulations in Diffusion-MRI: Effect of the substrate complexity and parameter choice on the reproducibility of results. Frontiers in Neuroinformatics, 14, 8.
Close, T. G., Tournier, J.-D., Calamante, F., Johnston, L. A, Mareels, I., & Connelly, A. (2009). A software tool to generate simulated white matter structures for the assessment of fibre-tracking algorithms. NeuroImage, 47(4), 1288–1300. https://doi.org/10.1016/j.neuroimage.2009.03.077 parameter choice on the reproducibility of results. Frontiers in Neuroinformatics, 14, 8.
Alexander, D. C., Hubbard, P. L., Hall, M. G., Moore, E. a., Ptito, M., Parker, G. J. M., & Dyrby, T. B. (2010). Orientationally invariant indices of axon diameter and density from diffusion MRI. NeuroImage, 52(4), 1374–1389.






